Launch of MIRIAD Kit
GD Biotech is pleased to announce the launch of its MIRIAD kit with SAFE®Complete Care Competence MIRIAD is a multiplex…

Multiplex Immunoassay for Rodents Infectious and Animal Diseases
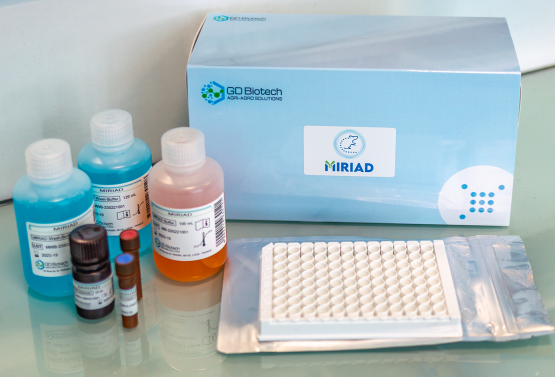
MIRIAD kit is declined in two versions, corresponding to the FELASA recommendations (see below).
VIRUSES (MOUSE)
Mouse Hepatitis Virus (MHV)
Murine Norovirus (MNV)
Minute Virus of Mice (MVM)
Mouse Parvovirus MPV1 & MPV2 (MPV)
Epizootic Diarrhea of Infant Mice (EDIM)
VIRUSES (MOUSE AND RAT)
Theiler’s Murine Encephalomyelitis Virus GD VII (TMEV)
Pneumonia Virus of Mice (PVM)
VIRUSES (RAT)
Rat Parvovirus (RPV)
Rat Minute Virus (RMV)
Rat Coronavirus/Sialodacryoadenitis Virus (RCV/SDA)
Toolan’s H-1 Virus (THV)
Kilham Rat Virus (KRV)
BACTERIA (MOUSE AND RAT)
Clostridium piliformis /Tyzzer’s Disease (TD)
Mycoplasma pulmonis (MPUL)
VIRUSES (MOUSE)
Mouse Hepatitis Virus (MHV)
Murine Norovirus (MNV)
Minute Virus of Mice (MVM)
Mouse Parvovirus MPV1 & MPV2 (MPV)
K virus (KV)
Lymphocytic Choriomeningitis Virus (LCMV)
Mousepox (ectromelia) Virus (ECTV)
Mouse Cytomegalovirus (MCMV)
Epizootic Diarrhea of Infant Mice (EDIM)
Mouse Polyomavirus (POLY)
VIRUSES (RAT)
Rat Parvovirus (RPV)
Rat Minute Virus (RMV)
Rat Coronavirus/Sialodacryoadenitis Virus (RCV/SDA)
Toolan’s H-1 Virus (THV)
Kilham Rat Virus (KRV)
VIRUSES (MOUSE AND RAT)
Theiler’s Murine Encephalomyelitis Virus GD VII (TMEV)
Mouse Adenovirus type 1 (FL)
Mouse Adenovirus type 2 (K87)
Pneumonia Virus of Mice (PVM)
Reovirus type 3 (REO)
Sendai Virus (SEV)
Hantavirus (HTNV)
PARASITES (MOUSE AND RAT)
Encephalitozoon cuniculi (ECUN)
BACTERIA (MOUSE AND RAT)
Clostridium piliformis /Tyzzer’s Disease (TD)
Mycoplasma pulmonis (MPUL)
Cilia-associated respiratory bacillus (CARB)
Health monitoring of laboratory rodents is essential to support animal welfare, as well as to prevent zoonotic risks – mandatory under some legislation. Poor health status can also be a source of experimental bias, impact on the reproducibility of experiments, and increase the number of animals used to an extent that is not compatible with the 3Rs principle (Replace, Reduce, Refine). Many agents – usually pathogens – are regularly tested in accordance with FELASA recommendations.
We have developed MIRIAD, a multiplexed ELISA serological test, for rodent health control in accordance with the 3Rs principle. The multiplexed ELISA is easy to implement and use without heavy investments, as it requires standard skills and equipment, which are already present in most animal facilities and testing centres.
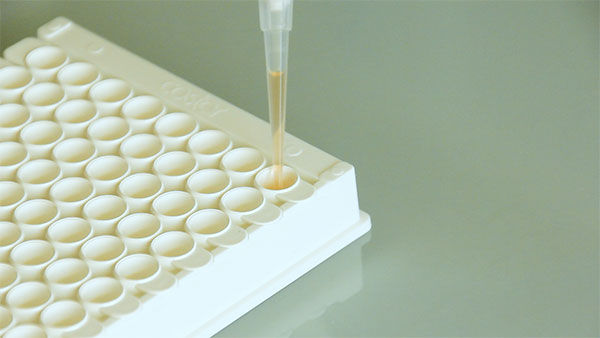
This test aims to improve the flexibility and reactivity of rodent health monitoring and represents an alternative to outsourcing the analysis. In addition, the multiplex format allows for the simultaneous detection of antibodies targeting different pathogens per assay.
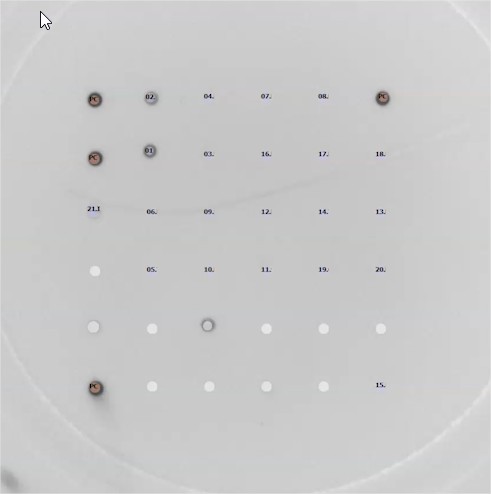
This approach is made possible by the miniaturisation of a probe array at the bottom of the wells of a 96-well plate. Parallelization allows for increased throughput with minimal human resources, and reduced costs and sample consumption to limit animal sacrifice. By increasing the frequency of testing and reducing the time required to obtain results at a competitive cost, the MIRIAD test can contribute to the early detection and management of serological agents recommended by FELASA.

GD Biotech is pleased to announce the launch of its MIRIAD kit with SAFE®Complete Care Competence MIRIAD is a multiplex…

Rémi Malbec, Responsable du développement de l’innovation chez GD Biotech, sera présent lors du 47° Colloque AFSTAL qui se déroulera…